This tool has been developed within the framework of the EULAC-PerMed project. It aims to provide information on regulatory and funding agencies regarding clinical trials in Latin America and Europe, and to facilitate the establishment of partnerships in clinical trials on personalised medicine across countries and regions.
If you are a clinical researcher interested in establishing partnerships or collaborating with ongoing projects on personalized medicine research, reach out to us at helpdesk![]() www.eulac-permed.eu with your contact information, your laboratory’s information, your areas of interest in personalised medicine, and if you are actively looking for partners for multinational clinical trials.
www.eulac-permed.eu with your contact information, your laboratory’s information, your areas of interest in personalised medicine, and if you are actively looking for partners for multinational clinical trials.
The information we provide on regulatory/funding requirements is based on past surveys conducted in collaboration with the participants of the project, and encompasses selected countries from Latin America and Europe. It is not exhaustive, but will continue to be updated.
- Regulatory requirements for clinical trials
- Information on funding agencies for clinical trials
- Mapping areas of interest in personalized medicine
| Country | Brief description of the regulatory framework for clinical research | Main competent authority to consult for clinical study authorization | Main authority to consult for marketing authorization | Other regulatory bodies that must approve clinical studies |
| Brazil | Clinical regulation is composed of a broad series of laws, rules and resolutions, and a compendium of these is produced and made publicly available by the National Health Surveillance Agency/ Agência Nacional de Vigilância Sanitaria (ANVISA). | National Health Surveillance Agency /Agência Nacional de Vigilância Sanitária – ANVISA | National Health Surveillance Agency /Agência Nacional de Vigilância Sanitária – ANVISA | Brazilian Registry of Clinical Trials / Registro Brasileiro de Ensaios Clínicos (ReBEC) |
| National Research Ethics Committee / Comissão Nacional de Ética em Pesquisa – CONEP | ||||
| Spain | Regulation for clinical trials in Spain is inscribed in the Royal Decree 1090/2015. Furthermore, the Law 14/2007 on Biomedical research outlines other key principles to be applied to clinical research. | Spanish Agency for Medicines and Medicinal Products / Agencia Española del Medicamento y Productos Sanitarios (AEMPS) | Spanish Agency for Medicines and Medicinal Products / Agencia Española del Medicamento y Productos Sanitarios (AEMPS) | Accredited ethics committee for research on drugs / Comités de Ética de la Investigación con medicamentos |
| Registro Español de Estudios Clínicos / Spanish Registry for Clinical Trials | ||||
| Chile | The principles and the overarching framework for clinical research in Chile are inscribed in the 20.120 Law on Scientific Research in Human Subjects, Their Genome and the Prohibition of Human Cloning and in the Technical Norm 57 – Regulation for the Execution of Clinical Trials that Utilize Pharmaceutical Products in Human Beings | Instituto de Salud Publica / Public Health Institute | Instituto de Salud Publica / Public Health Institute | National accredited scientific ethics committees / Comités de ética cientificos autorizados |
| Argentina | Clinical research regulation is inscribed in several of legal provisions: ANMAT Disposition No. 969/97 on the Applicable Regime to the Clinical Studies for Medical Devices *** ANMAT Disposition No. 6677/10 on Good Clinical Practices Regime for Clinical Pharmacological Studies *** MH Resolution No. 1480/11 on Guidelines for Clinical Investigations on Human Beings | Comité de Ëtica Enrique T. Segura IBYME-CONICET | National Administration of Drugs, Foods and Medical Devices / Administración Nacional de Medicamentos, Alimentos y Tecnología Médica (ANMAT) | National Registry of Persons / Registro nacional de las personas (RENAPER) |
| Approval of patient information sheets and consent forms by the National Authority for Data Protection | ||||
| Independent ethics committees / Comités Independientes de Etica | ||||
| Peru | The Regulatioin for Clinical Trials for Peru is stipulated in the Supreme Decree N° 021-2017-SA. | Instituto Nacional de Salud/National Institute of Health | Directorate General of Drug Supplies and Drugs / Direccion General de Medicaments Insumos y Drogas (DIGEMID) | Peruvian Clinical Trial Registry / Registro Peruano de Ensayos Clinicos (REPEC) |
| Costa Rica | The Regulatory Law on Biomedical Research defines the regulatory framework for clinical research in Costa Rica. | National Health Research Council / Consejo Nacional de Investigación en Salud (CONIS) | Ministry of Health / Ministerio de Salud | Scientific Ethics Committees /Comité Éticos Científicos (CEC) certified CONIS |
| Panama | Clinical research is regulated by Decree Law 84 of May 14, 2019, Decree 512 of June 28, 2019, and Ministerial Act 400 of June 7, 2021. These laws are enforced by the Ministry of Health, and all documents and procedures can be found here. | Ministry of Health / Ministerio de Salud | Ministry of Health / Ministerio de Salud | — |
| Uruguay | Research on human subjects, including clinical research is regulated by Decree 379/008 and Ministerial Ordinance 827/016. Furthermore, Decree 189/98 provides the national framework for the application of ICH GCP guidelines, and Decree 158/019 provides the framework for ethics committees overseeing research. All documents can be found here. | Ministerio de Salud Pública / Ministry of Public Health | Ministerio de Salud Pública / Ministry of Public Health | Institutional ethics committees where the research will be undertaken, and in particular cases the National Ethics Commission for Research / Comision Nacional de Etica en Investigacion. |
| Italia | Authorisation of clinical trials is regulated by Legislative Decree 211/2003, and its corresponding amendments. The Ministerial Decrees of May 12 2006 and February 8 2013 detail the requirements of ethics committees, while insurance requirements are set out in the Ministerial Decree of July 14 2009. | The competent authority for all clinical trials from Phases I to IV is the Italian Medicines Agency (AIFA) | Italian Medicines Agency / Agenzia Italiania del Farmaco | “The National Observatory on Clinical Trials (OsSC) manages the authorisation process of clinical trials (Phase I-IV) that are conducted in Italy, and provides a real time picture of the clinical research progress in the country”. |
| Clinical trials must be approved by validated ethics committees. The National Coordination Center for Ethical Territorial Committees coordinates, guides and monitors the evaluation activities of ethical aspects related to experimentations for clinical trials on medicinal products for human use and on medical devices, performed by the forty territorial ethics committees. | ||||
| Medical devices are regulated by the Ministry of Health Directorate General for Medicines and Medical Devices using the National Health Information System (NSIS). |
| Country | Main national funding body for clinical research | Other local funding bodies that can support PM research |
| Brazil | Ministry of Health / Ministério da Saude | National Research Council / Conselho Nacional de Pesquisa – CNPq |
| State foundations for research / Fundaçaoes Estaduais de Amparo à Pesquisa | ||
| Foundation for the assistance of research of the State of Minas Gerais / Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) | ||
| Foundation for the assistance of research of the State of Sao Paolo / Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) | ||
| Spain | Health Institute Carlos III/Instituto de Salud Carlos III | Ministry of Economic Affairs and Digital Transformation / Ministerio de asuntos económicos y transformación digital |
| Chile | National Agency for Research and Development / Agencia Nacional de Investigación y Desarrollo (ANID) | Corporation for the promotion of production / Corporacion de fomento a la produccion (CORFO) |
| Argentina | National Agency for the Promotion of Research, Technological Development and Innovation / Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (ANPCYT) | Sales Foundation / Fundación Sales |
| National Council of Scientific and Technical Research / Consejo Nacional de Investigaciones Científicas y Técnicas | ||
| Susan Komen Foundation/ Fundacion Susan Komen | ||
| University of Buenos Aires / Universidad de Buenos Aires, | ||
| Garrhan Pediatric Hospital / Hospital Pediatrico Garrahan, | ||
| National Cancer Institute / Instituto Nacional del Cáncer | ||
| Peru | Ministry of Health of the Nation / Ministerio de Salud de la Nación | San Agustín de Arequipa University / Universidad Nacional de San Agustín de Arequipa |
| San Marcos University / Universidad San Marcos | ||
| Cayetano Heredia University / Universidad Cayetano Heredia | ||
| National Council for Science, Technology and Scientific Innovation / Consejo nacional de Cienca, Tecnologia e Innovacion Tecnologica (CONCYTEC) | ||
| Alemania | German Research Foundation / Deutsche Forschungsgemeinschaft (DFG) | German Cancer Aid / Deutsche Krebshilfe (DKH) |
| Federal Ministry of Education and Research / Bundesministerium für Forschung und Bildung (BMBF) | ||
| Costa Rica | Fondo de Investigación e Innovación Tecnológica (FIIT) / Research and Technological Innovation Fund | Consejo Nacional para Investigaciones Científicas y Tecnológicas (CONICIT)/National council for Scientific and Technological Research |
| Italy | Ministry of Health | Cariplo Foundation / Fondazione Cariplo |
| AIRC Foundation for Cancer Research in Italy / Fondazione AIRC per la Ricerca sul Cancro (AIRC) | ||
| Lombardy Region / Regione Lombardia | ||
| Panama | National Secretariat of Science, Technology and Innovation/ SECRETARIA NACIONAL DE CIENCIA, TECNOLOGÍA E INNOVACIÓN | — |
| Uruguay | National Agency for Research and Innovation / Agencia Nacional de Investigacion e Inovacion (ANII) | Sectorial Commission for Scientific Researc/ Comision Sectorial de Investigacion Cientifica (CSIC) |
International organizations and programs that can fund PM research in LAC and EU countries:
- European Commission
- ERA PerMed
- NIH
The findings of a dedicated survey have been summarized here, presenting the main areas of interest for collaborations in clinical trials in Personalised Medicine. The survey was carried out in December 2020, and was circulated to stakeholders, participants, and potential collaborators of the EULAC PerMed project.
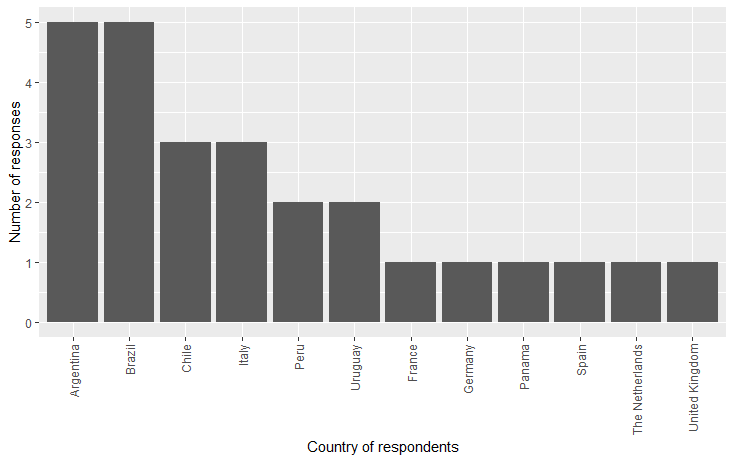
A long version of the survey aimed at collecting information on areas of interest in research or ongoing projects related to Personalised Medicine, and a shorter version was focused on regulatory and funding information. A total of 28 participants answered the survey (26 for the long version, 2 for the short version). Most of the respondents were from Argentina and Brazil (five from each country). In total, eight (30.8%) of the respondents were from Europe, with the majority of the participants being from Italy (three).
Figure 1. Countries of the survey’s participants.
Areas of interest in personalized medicine research
We asked participants to list a maximum of five areas of interest in personalized medicine research, and five potential areas of collaboration in personalized medicine research. Since these were open questions, we generated word clouds to summarize the participants’ responses using R’s ‘wordcloud’ package. We analyzed responses for all participants, for respondents from Latin America and for those from Europe.
Based on our results, cancer, bioinformatics, genomics, machine learning, artificial intelligence, and health systems were prioritized by participants as areas of interest in personalized medicine research (Figure 2). Similar results were observed for participants from Latin America (Figure 3), whereas the areas of interest identified by participants from Europe were cancer, radiomics, bioinformatics, epidemiology, imaging, modelling, and machine learning (Figure 4).
Figure 2. Areas of interest in personalized medicine research, all participants
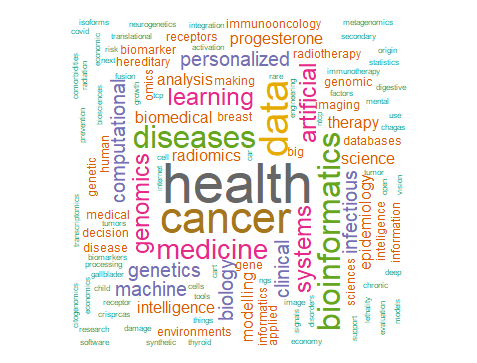
Figure 3. Areas of interest in personalized medicine research (participants from Latin America)

Figure 4. Areas of interest in personalized medicine research (participants from Europe)
Potential areas of collaboration between Latin America and Europe
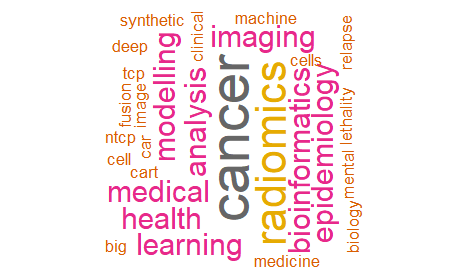
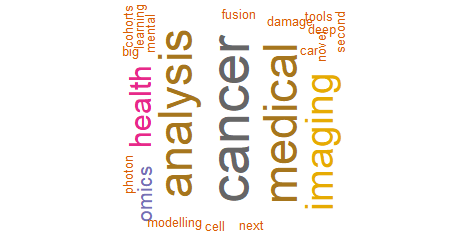
When asked about potential areas of collaboration across regions, the areas most frequently mentioned were health data, cancer, genetics, machine learning, artificial intelligence, imaging, sequencing, and rare diseases (Figure 5). Responses from participants from Latin America identified similar potential areas of collaboration. Genomics, and analysis of genetic variants were also areas frequently identified by participants from this region (Figure 6). On the other hand, according to participants from Europe, potential areas of collaboration in personalized medicine were cancer, data analysis, medical imaging, and omics (Figure 7).
Figure 5. Potential areas of collaboration in personalized medicine research, all participants

Figure 6. Potential areas of collaboration in personalized medicine research (participants from Latin America)

Figure 7. Potential areas of collaboration in personalized medicine research (participants from Europe)
We would like to thank researchers from the following institutions for their input:
- Data Extreme Lab / National Laboratory of Scientific Computing (Brazil), http://dexl.lncc.br
- Computational Systems Biology Laboratory, University of Sao Paulo (Brazil), https://www.csbiology.org/
- Biomedical Imaging Group Rotterdam, University Medical Center Roterdam (The Netherlands), https://bigr.nl/
- Centre for Translational Bioinformatics at the William Harvey Research Institute, Queen Mary University of London (United Kingdom), https://www.qmul.ac.uk/c4tb/
- Laboratoire Traitement du Signal et de l’Image, Université de Rennes 1 (France), https://www.ltsi.univ-rennes1.fr/
- Laboratory of Genetic and Molecular Analysis, University of Campinas (Brazil), https://lagm.cbmeg.unicamp.br/en/quemsomos.html
- Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST/IRCCS; Italy), https://www.irst.emr.it/it/
- Escola Nacional de Saude Publica, FIOCRUZ (Brazil), http://ensp.fiocruz.br/
- Center for Omics Sciences, San Raffaele Scientific Institute (Italy), https://research.hsr.it/en/centers/omics-sciences.html
- The State Funding Agency of Minas Gerais (FAPEMIG; Brazil), https://fapemig.br/
- The Gonçalo Moniz Institute of FIOCRUZ https://www.bahia.fiocruz.br/
- University of Concepcion (Chile), https://www.udec.cl/
- Departamento de Informática en Salud y Servicio de Patologia, Hospital Italiano de Buenos Aires (Argentina), https://www.hospitalitaliano.org.ar/#!/home/infomed/inicio
- Departamento de Investigación e Innovación, Parc Sanitari Sant Joan de Déu (Spain), https://www.irsjd.org/es/investigacion/investigacion-en-red/
- Centro de Genética y Genómica, Instituto de Ciencias e Innovación en Medicina, Facultad de Medicina, Clínica Alemana Universidad del Desarrollo (Chile), https://medicina.udd.cl/icim/
- Bioscience Data Mining Group, CIDIE – UCC – CONICET (Argentina)
- Unidad ETESA UC, Pontificia Universidad Católica de Chile (Chile), https://facultadmedicina.uc.cl/centros-y-programas/investigacion-clinica-uc/unidad-etesa-uc/
- Unidad de Bioinformática, Institut Pasteur de Montevideo (Uruguay), http://pasteur.uy/en/research/units/bioinformatic/
- Laboratorio de Carcinogénesis Hormonal, IBYME-CONICET (Argentina), https://www.ibyme.org.ar/laboratorios/15/carcinogenesis-hormonal
- Departamento de Ingeniería de Sistemas e Informática, CiTeSoft, Universidad Nacional De San Agustín de Arequipa (Peru), https://fips.unsa.edu.pe/departamento-academico-de-ing-de-sistemas-e-informatica/
- Dirección de Investigación en Salud, Instituto de Evaluación de Tecnologías en Salud e Investigación – Seguro Social – ESSALUD (Peru), http://www.essalud.gob.pe/ietsi/
- Institute of Medical Biometry and Informatics / Statistical Genetics Research Group , University of Heidelberg, (Germany), https://www.klinikum.uni-heidelberg.de/en/medizinische-biometrie/forschung/arbeitsgruppen/statistical-genetics
- Grupo de Investigaciones en Biotecnología, Bioinformática y Biología de Sistemas (GIBBS), Universidad Tecnológica de Panamá (Panama), http://biotecnologia.utp.ac.pa/
- Laboratorio de Bioinformática y Genómica, Departamento de Química Biológica, Universidad de Buenos Aires, (Argentina), http://www.qb.fcen.uba.ar/
- Sección de Genética Clínica, Facultad de Medicina, Universidad de la República (Uruguay), http://www.genetica.fmed.edu.uy/
